The news of the Ebola vaccine trial in Hohoe in the Volta region of Ghana began to filter into the media in May. The trial was scheduled to take place at the Hohoe Midwifery Training College. The participants were promised 200 Ghana cedis and a mobile phone to take part in the vaccine trial. This has created a pug fest in the media and in Ghana about the sensibility or otherwise of having this vaccine trial in Ghana. Let us recall that that as of May 18 2015 of the 26,763 cases of Ebola in the worst hit countries of Guinea, Liberia and Sierra Leone with about 11,074 confirmed deaths a very high death rate. Let us analyze some background information about the virus and its devastating potential.
Ebola as a virus is classified as a Biosafety Level 4 microbe by the Center for Disease Control (CDC) of the USA. This is actually a quote from their website:
BSL-4 builds upon the containment requirements of BSL-3 and is the highest level of biological safety. There are a small number of BSL-4 labs in the United States and around the world. The microbes in a BSL-4 lab are dangerous and exotic, posing a high risk of aerosol-transmitted infections. Infections caused by these microbes are frequently fatal and without treatment or vaccines. Two examples of microbes worked with in a BSL-4 laboratory include Ebola and Marburg viruses.
In fact in Russia, Ebola virus is handled in Russia’s Vector Research located in the environs of the city of Novosibirsk in Siberia far from human settlements. In fact this is what the Siberian Times of 15’th October 2014 says about Ebola at the Vector institute:
Founded in 1974, the Vector Center is arguably the world’s most eminent facility for studying Ebola. It is one of just two institutions in Russia where special conditions are provided for examining the virus, which is considered most dangerous in the world.
This is the nature of the virus that we are dealing with. Note the use of the words special conditions are provided for examining the virus in the excerpt from the Siberian Times at the Vector Institute in Siberia.
Note also the use of the words Infections caused by these microbes are frequently fatal and without treatment or vaccines on the website of the CDC in the United States.
The reason for stating these two sources from America and Russia is that these two countries have the most advanced research in bioweapons research and the study of viruses as a possible use as a bioweapon. So it is relevant to our thinking to understand what we are dealing with here.
The Ebola vaccine trial in Ghana which was lobbied for by a member of parliament and was supposed to be conducted in conjunction with the Food and Drug Board (FDB) and the new University of Health and Allied Sciences. We are told that it is a phase 1 trial with about 18 participants to be selected in Hohoe.
One professor Alex Dodoo at the University of Ghana Medical School as strongly lobbied for the phase 1 vaccine trial. Due to the strong public outcry, the minister of health on June 10 2015 ordered the vaccine trial to be stopped. Parliament also jumped into the fray calling for a moratorium on the vaccine trial. The Ghana Academy of Arts and Sciences also jumped into the fray with this statement below:
In January this year, the Ghana Academy of Arts and Sciences became aware, from a newspaper report, that a clinical trial for an Ebola Virus Disease (EVD) vaccine was due to start in Ghana before the end of March.
Given the uncertainties about the nature of the Ebola virus and risks in clinical trials, the Academy set up a 5-person Technical Committee made up of Fellows of the Academy to undertake an urgent review of the matter and report to the Academy.
In its preliminary report, the Committee noted, among other things, that the proposal before the Food and Drugs Authority (FDA) was for a Phase II clinical trial of an Ebola Virus Disease (EVD) vaccine, developed by GlaxoSmithKline/US National Institutes of Health (NIH).
Such an undertaking must be preceded by a thorough evaluation of the available data, and the application subjected to the appropriate procedures.
On the basis of its preliminary investigation and study, the Committee recommended a second look at the design of the study; a review of the basis for the selection of Ebola-unaffected countries like Ghana; and, because some Ghanaians have anti-bodies to the adenovirus, a fuller understanding of the adenovirus vector used in the development of the test vaccine.
In discharge of the Academy’s mandate to provide independent science-based advice for policy making, the Council of the Academy asked the President of the Academy to bring these concerns urgently to the attention of the Minister of Health.
This was immediately done, attaching a copy of the preliminary report of the Committee and confirming the Academy’s preparedness to provide all necessary support to the Ministry in dealing with this critical matter. After some delay, the newly-appointed Minister of Health convened a meeting on June 03, at which the concerns and issues raised by the Academy were discussed with the technical staff of the Ministry of Health (MOH), the Food and Drugs Authority and its expert advisors, as well as the Principal Investigators in the GSK/NIH Phase II trials.
The main concerns raised by the Technical Committee of the Academy relate to the following:
- Major uncertainties about a. the nature and origins of the Ebola virus, including the circumstances of its appearance in Guinea;
- Whether the Zaire strain of the virus, which is the one being used in the GSK vaccine to be tested in Ghana, is the strain responsible for the Ebola epidemics in Liberia, Mali, Nigeria, Senegal and Sierra Leone, and c. the identity and characteristics of other strains of the Ebola virus that might exist;
- The use in the GSK/NIH vaccine of a gene particle of the wild species of the Zaire Ebola virus, rather than the gene particle of the Makona strain isolated in the epidemic in Guinea;
- What pre-clinical animal experimentations had been carried out with a vaccine based on the Makona strain to establish evidence of safety, immunogenicity and protection?
- What basis is there for expecting that immune responses generated against the wild type Zaire Ebola virus GSK vaccine formulation (construct), with a live non-replicating chimpanzee adenovirus carrying a gene from Zaire Ebola virus, would be effective against the Makona strain or any other Ebola virus species and strains?
- After a test vaccine has been shown in the vaccinated individual to produce an immune response (immunogenicity), what guarantee would there be, in this instance, that the vaccine would offer protection against the full Zaire Ebola virus and other species and strains?
- On the basis of research conducted so far towards vaccine development, what is the likelihood of the present construct of vaccines protecting communities against the rapid emergence of new, more virulent strains of the virus, as appears to have happened with the Makona: the risk of false confidence deriving from the use of a new vaccine must be noted;
- What assurances do we have that the chimpanzee-derived live adenovirus vector used in the GSK vaccine construct, although non-replicating for now, will remain dormant and not itself cause a disease to compromise the health of the people of Ghana?
- It is to be noted that the application for the GSK Ebola vaccine Phase II trial in Ghana includes children, even though the Phase I trial in the US, UK, Mali and Switzerland was limited to adults, raising the question of dosage profiles for children and other vulnerable groups in the Phase II trial;
- What evidence is there of strict compliance with the The International Committee on Harmonization Protocol Guidelines for Clinical Trials, including full “informed consent” by all volunteers?
It was confirmed at the above-mentioned meeting between the President and Technical Committee of the Ghana Academy of Arts and Sciences, and staff of the MOH, the Food and Drugs Authority (FDA) and its expert advisors, and the Principal Investigators in the GSK/NIH Phase II trial, that the processes for the approval of the Phase II clinical trial of the GSK Ebola Virus Disease test vaccine had not been concluded.
Our firm understanding was that the approval process will continue to take into account the concerns and issues raised by the Academy.
In the course of the meeting, it was mentioned approval had already been given to an application for a separate Phase I trial in Hohoe, of a test vaccine with a different construct from the GSK test vaccine, which latter had been the focus of concern of the Academy.
This came as a shock to the Academy representatives at the meeting, as nothing had been said anywhere previously about a separate Phase I clinical trial application, let alone its approval.
The Academy’s representatives therefore refused to discuss that matter.
However, it is to be noted that the Phase I trial of the GSK vaccine in Europe produced an adverse event, namely, prolonged bleeding, in 10% – 15% of the vaccinated population.
This is a serious adverse event that calls for extreme caution in approving clinical trials, both Phase I and Phase II, in the country.
Moreover, it is the case that those vaccinated at Phase I and Phase II may be shedding the adenovirus vector into the surrounding community.
In the absence of a map of adenovirus prevalence in the trial sites, there is a high risk of an ‘escape virus’ merging with the endemic adenoviruses to create more virulent strains.
For that reason alone it is important that the exposed communities and, indeed, the general public be adequately informed of such trials and their benefits and risks.
In conclusion, The Ghana Academy of Arts and Sciences wishes to state its firm position that, subject to satisfactory answers to the issues it has raised, and considering the gaps in our knowledge and state of preparedness, it would be unsafe to undertake the proposed EVD vaccine clinical trials in Ghana.
The Academy affirms its availability to help the Ministry of Health, the Food and Drugs Authority (FDA) and other parties involved in the approval process to arrive at sound, independent decisions on this and other critical matters facing the country.
[The Ghana Academy of Arts and Sciences, with a membership of 111 Fellows drawn from all fields of learning, has from its inception in 1959 been charged with a key role in thought leadership and making inputs into policymaking through research and evidence-based advice.
Over the years efforts have been made, and continue to be made, to re-profile the Academy in the light of changing conditions]
Issued by
Honorary Secretary, Ghana Academy of Arts and Sciences
12 June 2015
Professor Dodoo came out to say that the suspension of the vaccine trial was a sad day for Ghana and not Ghana was losing the chance to earn billions of dollars from this trial and an opportunity to increase the GDP of the country. He mentioned the case of Switzerland which earns billions of dollars from its hi-tech pharma industry.
I do not question professor Dodoo’s medical expertise but I question his common sense and cultural dignity. How is Ghana going to earn billions of dollars from a vaccine trial involving about 18 people when the results of the trials will be taken away by the big pharma companies conducting the trials? All the Research and Development work after the trials to produce a workable vaccine will never be done in Ghana.
Therefore this vaccine trial cannot earn billions of dollars from this project. I do not know on which planet he is hallucinating on. There will be no transfer of technology, no biotechnology research institutions built in Ghana by the companies testing the vaccine in Ghana, no graduate and post-doctoral students trained to work in vaccine design and research in Ghana.
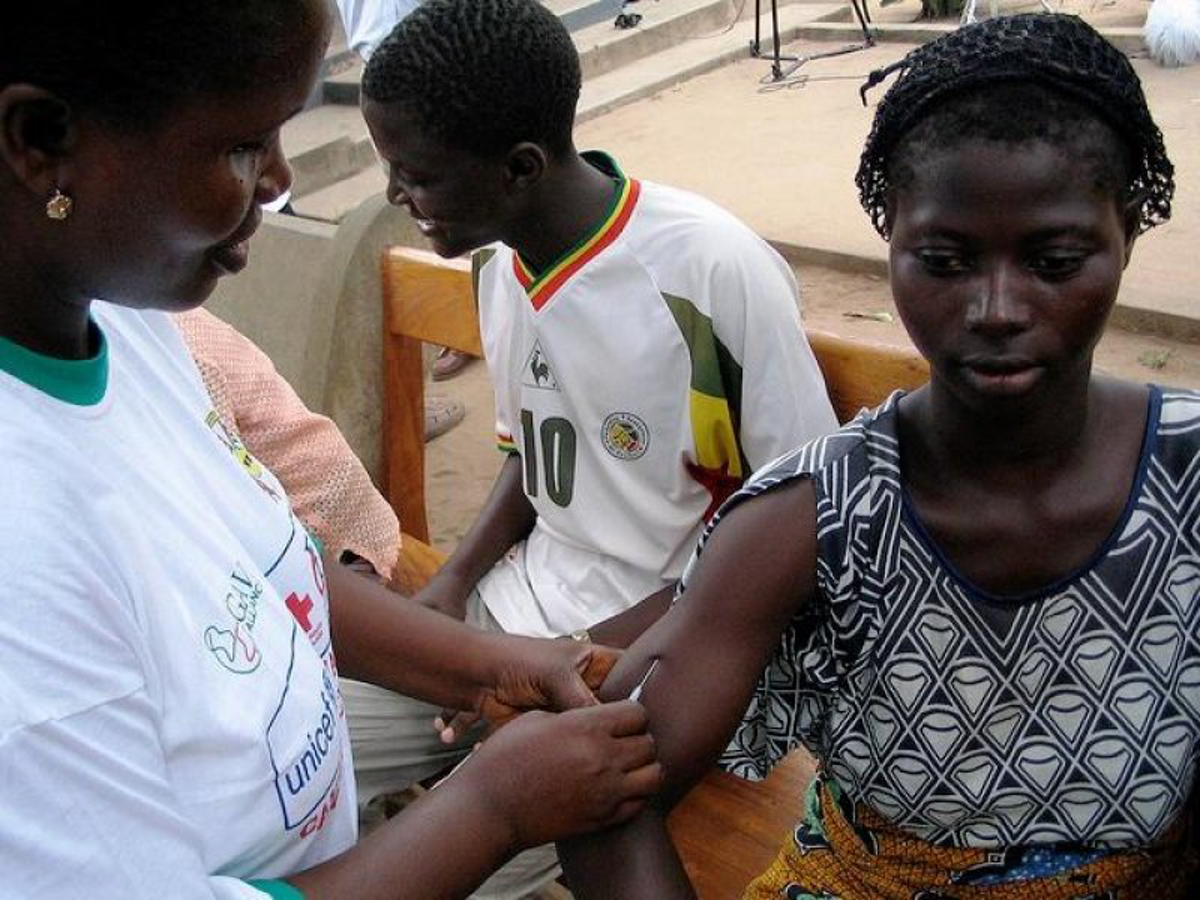
Let us now parse the paltry sum of 200 Ghana cedis (about $50) and a cheap mobile phone that the participants of the vaccine trial were going to be earning. We will contrast this with a similar trial of an Ebola vaccine conducted in the US. This is from a CNN online article of September 4 2015 which in part says:
The experimental vaccine, developed by the pharmaceutical company GlaxoSmithKline and the NIAID, is being given to the three healthy human volunteers at first to see if they suffer any adverse effects. If deemed safe, it will then be given to another small group of volunteers, ages 18 to 50, to see if it produces a strong immune response to the virus. All will be monitored closely for side effects…
The vaccine will be administered to volunteers by an injection in the deltoid muscle of their arm, first in a lower dose, then later in a higher dose after the safety of the vaccine has been determined.
Each volunteer is being paid approximately $1,700 for their time and inconvenience, the NIH said. The government agency expects to spend around $1.6 million on the trial overall.
I bolded the text of… Each volunteer is being paid approximately $1,700 for their time and inconvenience…
Now let us contrast this with the insulting sum of $50 being paid to the volunteers in Ghana. Observe that $1700 is 34 times the amount of $50! Therefore this metric seems to indicate that African volunteers are 34 times less valuable than white Americans.
Ebola as one of the most dangerous viruses known to man certainly needs to be suppressed through a cure or through the development of a vaccine when possible. We welcome that initiative. Many countries like Canada, Japan, Russia and the US have been working on an Ebola vaccine and are planning or conducting human trials to test possible vaccines been developed.
What can and what should Ghana and Africa demand from requests for clinical trials for Ebola. We should have the following minimal demands;
- A multibillion dollar biopharma fund to develop higher education facilities, research institutions and training programs in the biosciences including vaccine research and development
- A comprehensive quarantine of Ebola vaccine trial participants beyond the minimum of 21 days for the effects of the Ebola virus to be felt. The quarantine should be far from population settlements in specialized isolated zones as we do not have the health care infrastructure to control and limit any outbreaks if they do happen. In fact mathematical models developed at the Vector institute in Siberia show that the number of contacts between the sick and healthy people should be at least halved to stabilize infection levels. So creating specialized isolation zones should be the first minimal step in any clinical trial in Ghana or elsewhere in Africa
- Vaccine trial participants should be paid exactly the same compensation given to vaccine trial participants in the western countries
- The sharing of all data and results from such trials
- The right to produce generic versions of the vaccines that might be developed as a result of the clinical trials conducted in Ghana and elsewhere in Africa.
We will end by quoting in full a revealing article on bioethics written in The British Medical Journal (BMJ) by professor Felix ID Konotey Ahulu Kwegyir Aggrey Distinguished Professor of Human Genetics University of Cape Coast, Ghana and Consultant Physician Genetic Counsellor in Sickle Cell and Other Haemoglobinopathies. This article was posted on the website of The BMJ on 14 June 2015(link: http://www.bmj.com/content/350/bmj.h2105/rr-7). The article in full is below:
Ebola and Ethics: Ghana Academy of Arts & Sciences and Ghana Parliament Suspend Ebola Vaccine Trials
Dr J K Anand [1] whom I have never met refers to me as the “Krobo tribesman who also happens to be a Fellow of the RCP London” [2] Indeed I am a Krobo Tribesman about which see later, but his mention that I am also Fellow of the Royal College of Physicians London makes me state if boastfully that I am also Fellow of other prestigious Colleges and Academies. The one I am most proud of this very day is the Ghana Academy of Arts and Sciences not just because they once gave me their GOLD MEDAL for “Outstanding Contribution to knowledge in Medical Science” the same evening as they gave the legendary Francis Allotey also GOLD MEDAL in Mathematical Sciences, but also that they could produce a Communique like this one (below) giving reasons why they prevailed on the Government of Ghana to suspend with immediate effect the Ebola Virus Vaccine Trials due to start in the country.
WORLD EXPERTS PLEASE STUDY THIS STATEMENT
I invite the most brilliant scientists in Johns Hopkins, Harvard, Oxford, Utrecht, NIH, CDC, and London School of Tropical Medicine & Hygiene to study this GAAS Communique from which they will derive much benefit as they go about their own vaccination programmes in Europe, USA, and elsewhere. I recommend it to the WHO too.
Below is the Academy’s full narration of events leading up to the announcement of the Ebola vaccine trial in Ghana [3]
In January this year, the Ghana Academy of Arts and Sciences became aware, from a newspaper report, that a clinical trial for an Ebola Virus Disease (EVD) vaccine was due to start in Ghana before the end of March.
Given the uncertainties about the nature of the Ebola virus and risks in clinical trials, the Academy set up a 5-person Technical Committee made up of Fellows of the Academy to undertake an urgent review of the matter and report to the Academy.
In its preliminary report, the Committee noted, among other things, that the proposal before the Food and Drugs Authority (FDA) was for a Phase II clinical trial of an Ebola Virus Disease (EVD) vaccine, developed by GlaxoSmithKline/US National Institutes of Health (NIH).
Such an undertaking must be preceded by a thorough evaluation of the available data, and the application subjected to the appropriate procedures.
On the basis of its preliminary investigation and study, the Committee recommended a second look at the design of the study; a review of the basis for the selection of Ebola-unaffected countries like Ghana; and, because some Ghanaians have anti-bodies to the adenovirus, a fuller understanding of the adenovirus vector used in the development of the test vaccine.
In discharge of the Academy’s mandate to provide independent science-based advice for policy making, the Council of the Academy asked the President of the Academy to bring these concerns urgently to the attention of the Minister of Health.
This was immediately done, attaching a copy of the preliminary report of the Committee and confirming the Academy’s preparedness to provide all necessary support to the Ministry in dealing with this critical matter. After some delay, the newly-appointed Minister of Health convened a meeting on June 03, at which the concerns and issues raised by the Academy were discussed with the technical staff of the Ministry of Health (MOH), the Food and Drugs Authority and its expert advisors, as well as the Principal Investigators in the GSK/NIH Phase II trials.
The main concerns raised by the Technical Committee of the Academy relate to the following:
- Major uncertainties about a. the nature and origins of the Ebola virus, including the circumstances of its appearance in Guinea;
- Whether the Zaire strain of the virus, which is the one being used in the GSK vaccine to be tested in Ghana, is the strain responsible for the Ebola epidemics in Liberia, Mali, Nigeria, Senegal and Sierra Leone, and c. the identity and characteristics of other strains of the Ebola virus that might exist;
- The use in the GSK/NIH vaccine of a gene particle of the wild species of the Zaire Ebola virus, rather than the gene particle of the Makona strain isolated in the epidemic in Guinea;
- What pre-clinical animal experimentations had been carried out with a vaccine based on the Makona strain to establish evidence of safety, immunogenicity and protection?
- What basis is there for expecting that immune responses generated against the wild type Zaire Ebola virus GSK vaccine formulation (construct), with a live non-replicating chimpanzee adenovirus carrying a gene from Zaire Ebola virus, would be effective against the Makona strain or any other Ebola virus species and strains?
- After a test vaccine has been shown in the vaccinated individual to produce an immune response (immunogenicity), what guarantee would there be, in this instance, that the vaccine would offer protection against the full Zaire Ebola virus and other species and strains?
- On the basis of research conducted so far towards vaccine development, what is the likelihood of the present construct of vaccines protecting communities against the rapid emergence of new, more virulent strains of the virus, as appears to have happened with the Makona: the risk of false confidence deriving from the use of a new vaccine must be noted;
- What assurances do we have that the chimpanzee-derived live adenovirus vector used in the GSK vaccine construct, although non-replicating for now, will remain dormant and not itself cause a disease to compromise the health of the people of Ghana?
- It is to be noted that the application for the GSK Ebola vaccine Phase II trial in Ghana includes children, even though the Phase I trial in the US, UK, Mali and Switzerland was limited to adults, raising the question of dosage profiles for children and other vulnerable groups in the Phase II trial;
- What evidence is there of strict compliance with the The International Committee on Harmonization Protocol Guidelines for Clinical Trials, including full “informed consent” by all volunteers?
It was confirmed at the above-mentioned meeting between the President and Technical Committee of the Ghana Academy of Arts and Sciences, and staff of the MOH, the Food and Drugs Authority (FDA) and its expert advisors, and the Principal Investigators in the GSK/NIH Phase II trial, that the processes for the approval of the Phase II clinical trial of the GSK Ebola Virus Disease test vaccine had not been concluded.
Our firm understanding was that the approval process will continue to take into account the concerns and issues raised by the Academy.
In the course of the meeting, it was mentioned approval had already been given to an application for a separate Phase I trial in Hohoe, of a test vaccine with a different construct from the GSK test vaccine, which latter had been the focus of concern of the Academy.
This came as a shock to the Academy representatives at the meeting, as nothing had been said anywhere previously about a separate Phase I clinical trial application, let alone its approval.
The Academy’s representatives therefore refused to discuss that matter.
However, it is to be noted that the Phase I trial of the GSK vaccine in Europe produced an adverse event, namely, prolonged bleeding, in 10% – 15% of the vaccinated population.
This is a serious adverse event that calls for extreme caution in approving clinical trials, both Phase I and Phase II, in the country.
Moreover, it is the case that those vaccinated at Phase I and Phase II may be shedding the adenovirus vector into the surrounding community.
In the absence of a map of adenovirus prevalence in the trial sites, there is a high risk of an ‘escape virus’ merging with the endemic adenoviruses to create more virulent strains.
For that reason alone it is important that the exposed communities and, indeed, the general public be adequately informed of such trials and their benefits and risks.
In conclusion, The Ghana Academy of Arts and Sciences wishes to state its firm position that, subject to satisfactory answers to the issues it has raised, and considering the gaps in our knowledge and state of preparedness, it would be unsafe to undertake the proposed EVD vaccine clinical trials in Ghana.
The Academy affirms its availability to help the Ministry of Health, the Food and Drugs Authority (FDA) and other parties involved in the approval process to arrive at sound, independent decisions on this and other critical matters facing the country.
[The Ghana Academy of Arts and Sciences, with a membership of 111 Fellows drawn from all fields of learning, has from its inception in 1959 been charged with a key role in thought leadership and making inputs into policymaking through research and evidence-based advice.
Over the years efforts have been made, and continue to be made, to re-profile the Academy in the light of changing conditions]
Issued by
Honorary Secretary, Ghana Academy of Arts and Sciences
12 June 2015
http://citifmonline.com/2015/06/12/govt-was-warned-against-ebola-vaccine…
– See more at: http://citifmonline.com/2015/06/12/govt-was-warned-against-ebola-vaccine…
CONGRATULATIONS TO GHANA ACADEMY OF ARTS AND SCIENCES
I do not honestly know what readers of the BMJ think after reading this but, I for one, give FULL Marks to the Ghana Academy of Arts and Sciences whose current President Professor Akilagpa Sawyerr, is an International Lawyer of no mean standing. I much congratulate the Scientific Section of our noble ACADEMY for their brilliance. Ayenyekoo! As my Mother Tongue says Congratulations to a Group.
KROBO TRIBAL SENSIBILITIES
There will be so much rejoicing in my tribe. Those who read The Lancet will remember the case of the Krobo Tribal Chief who posed a question that scientists in Harvard would find hard to answer [4]. After addressing a large gathering of chiefs, elders, women, and school children on the local epidemiology of what in our Krobo language, is called kpaanyor (meaning “eight” – the nearest acoustic rendering of AIDS in the language), when the floor was open for questions, and an illiterate chief enquired whether it was true a vaccine for AIDS was in preparation, and I said yes, he exclaimed “What? Are they going to prick us with needles so we can do what we like?” [4]
This chief has heard on the radio in the local language that Harvard University scientists, NIH, and CDC said eating African green monkey meat brought the AIDS calamity on the tribe when he knew full well that international prostitution killed his daughter who had gone to Abidjan in the sex trade, while her twin sister who stayed in school and refused to join the sex trade was hale and hearty [5]. And here was I, a fellow tribesman, telling them that a vaccine was on the way so they should take heart?
The present Paramount Chief of The entire Manya Krobo mega Tribe, Nene Sakite II, is a scholar. He speaks English better than many in England. He has read from CDC publications that Africans eating bats brought the West African Epidemic, but if even the Ghana Academy of Arts and Sciences had not come out as authoritatively as they have done our Nene Sakite II would NEVER have allowed anybody to go to the tribe and vaccinate them against Ebola Virus Disease, never mind what WHO advises.
PROFESSOR JONATHAN H ADDY’S UNASWERABLE QUESTION
In the same Lancet reference [4] responding to Professor PJ Weidle and colleagues [6] on HIV Vaccines for Africa I mentioned how at Korle Bu Teaching Hospital I discussed the continental tragedy with a professor of Medicine. Even before I mentioned vaccines he said to me “Look here, for a vaccine to be worth its name, it must produce antibodies. Would you surrender your seronegative status for a sero-positive one?’”[4] Would Ghanaians swap their Ebola Virus Antibody Negative status for a sero-Positive one? Professor Addy who discovered Ghanaian Essential Hypertension was of Mendelian Homozygous Recessive Inheritance [7] would not understand how scientists from abroad bypass such as him and the geniuses at the Ghana College of Physicians and Surgeons who scored Distinctions in their UK Universities (London, Glasgow, Sheffield, Cambridge), and quietly go to our Ministry of Health with Vaccination plans?
MORE THAN SCIENCE AND DANGEROUS
As Dr J K Anand implied in his comment “One can understand the pain of the share-holders of the Companies manufacturing the vaccine” [2], and there could be danger in obstructing programmes that would bring money to Drug Firms and share-holders. Readers of BMJ will remember my being given four Body Guards because I was unmasking a “scientific” untruth in the USA from which Insurance Companies were profiting [8]. Suspending the Vaccine Trials would hugely displease some powerful financial interests it may prove dangerous to stand in their way. How else does one explain my 4 body guards?
BUT PRESIDENT OBAMA WILL YOU NOT HELP US?
President Barack Obama is 50 per cent African. He mentioned the word “Ebola” this past week at the G7 Conference. As Mordecai told Queen Esther of the genocide plot of Haman to destroy the Jews “Who knows whether you have come to the kingdom for such a time as this?” [9], applying this Africa, who knows whether this remarkable President, the most powerful man in the world, has come at such a time as this?
Whenever we Africans reveal our suspicions about Conspiracy Facts we are shouted down with “CONSPIRACY THEORIES!” But as I said less than a fortnight ago in the BMJ [10] some of us Africans do not trust what is happening. BMJ’s Sophie Arie [11] wrote “Candidate treatments and vaccines for Ebola were developed only because the United States considered the virus a potential weapon for bioterrorism” The United States? Oh Mr President, only you can stop these laboratory tinkering with the nuclei of dangerous viruses! The Lancet predicted the BIOLOGICAL BOMB [12]. You yourself [13 14], and President Clinton [15] apologized openly for American scientific roguery. Please help us. O help us!
For myself, allow me to ask just two things of you before you leave Office next year, God willing, so that we do not forget you in Africa.
(1) Devise a means that can help us distinguish your good scientists the from the wicked ones who come in sheep’s clothing to (as Lord Richie Calder put it) promote “Public Health in reverse”.[12].
(2) Electrify as much of our Continent as possible with solar energy which we have in abundance. That will help us deal with the environment, drain the marshes, build proper drains to get rid of the mosquitoes and other preventable diseases. This will reduce drastically the need for vaccines. Divert your Vaccine Donations to this effect. We have, ourselves, begun looking for African versions of Bill Gates who will donate their Billions for Environmental Infrastructure rather than for Vaccines. Why do we go on vaccinating dirty children drinking dirty water?
The Population Control by hook or by crook Motto of some Donor Countries should not be associated with you, Sir. I myself am emphasizing Genetic Counselling and Family Size Limitation to reduce the incidence of Abnormal Haemoglobin Ailments like Sickle Cell Disease [16, 17]. Please, we beg you, let your Legacy for Africa be Ethical Research and Environmental Improvement with Solar Energy for almost every home. This measure alone can reduce numbers of those traversing the Mediterranean to Europe every week in their thousands.
Visit the NIH and find out what is happening, please. Close the Dirty Labs. I knew Dr Ruddy Jackson MD of NIH and CDC’s Dr A N Schechter MD. Those were great American scientists doing marvelous work. Not like the one we unmasked at Ho in Ghana, caught red-handed by Mawuli Secondary School practicing “Public Health in Reverse” and sent back to the USA. Nor were Jackson and Schechter like the 4 Scientists whom America’s charming Ambassador in Ghana Her Excellency Shirley Temple Black forbade stepping foot in Ghana because they were bringing us drugs that your scientists proved to be useless, Oh Mr President help us before you leave Office. Thank You Sir!
FINALLY THANKS TO THE BRITISH MEDICAL JOURNAL
Finally I thank the BMJ, the world’s leading journal, for letting the Ghana Academy of Arts and Sciences educate the rest of the world. I do not know any other medical journal globally that will let Africans teach everybody else. Some rival front runners with the BMJ in Medical Journalism do not like it and reject our articles when an African criticizes a European who writes (Tafracher [18]) “scientific” untruths. But you have allowed us to present the very impressive Protocol that Ghana has used to detect serious flaws in the Ebola Vaccine programme. This Communique from Ghana may well be referred to in other countries as “The GAAS Vaccine Investigative Protocol” Thank you Dr Fiona Godlee for helping Africa.
Conflict of Interest: I am a Krobo Tribesman concerned for Clean Science and Ethics.
Felix I D Konotey-Ahulu FGA (Fellow of Ghana Academy of Arts & Sciences)
[University of Cape Coast, Ghana and 9 Harley Street, London W1G 9AL]
1 Anand JK. Ebola and Ethics: autopsy of a failure. Dr Konotey-Ahulu’s response BMJ Rapid Response 11 June 2015 www.bmj.com/content/350/bmj.h2105/rr-6
2 Anand JK. Ebola and Ethics. Are vaccine trials going on somewhere in Africa? BMJ Rapid Response June 1 2015. www.bmj.com/content/350/bmj.h2105/rr-4
3 Ghana Academy of Arts & Sciences Communique 12 June 2015 Full narration of events leading up to the announcement of the Ebola vaccine trial in Ghana.
http://citifmonline.com/2015/06/12/govt-was-warned-against-ebola-vaccine…
– See more at: http://citifmonline.com/2015/06/12/govt-was-warned-against-ebola-vaccine…
4 Konotey-Ahulu FID. AIDS in Africa. Lancet; 360: 1424 (2 November 2002)
5 Konotey-Ahulu FID. What Is AIDS? T-AD Co Watford 1989/1996, pages 141-143.
- Weidle PJ, Mastro TD, Alison DG, Nkengasong J, Machara D. HIV/AIDS. Treatment and HIV vaccines for Africa. Lancet 2002; 359: 2261-67.
7 Addy JH. Ghanaian essential hypertension in homozygous recessive inheritance. Lancet Aug 8 1992 pp 377-378, and Ghana Medical Journal 1990; 24: 164-169
8 Konotey-Ahulu FID. Four body guards and the perils of unmasking scientific truths. BMJ 2007; 335: 210-211 July 28. :
9 Mordecai to Esther: “Who knows whether you have come to the kingdom for such a time as this?” ESTHER chapter 4 verse 14“
10 Konotey-Ahulu FID. Ebola and ethics: “Are vaccine trials going on somewhere in Africa?” BMJ Rapid Response 2 June 2015 www.bmj.com/content/350/bmj.h2015/rr-5
11 Arie Sophie. Ebola: A game change for vaccines, or a scare that will soon be forgotten? BMJ 2015; 350: h1938
12 Lancet Annotations. The Biological Bomb. Lancet 1968 (March 20) Volume 1: p 465
13 Tanne Janice Hopkins. President Obama apologises to Guatemala over 1940’s syphilis study. BMJ 2010; 341.c5494. October 9, page 750
14 Konotey-Ahulu FID. President Obama apologises over study: International Guatemala co-operative research and practice in jeopardy. BMJ Rapid Response 4 October 2010
15 Clinton President WJ. Apology on br-Gaultehalf of the American Government to 8 survivors of the Tuskegee Syphilis Experiment victims. World-wide Radio and Television PBS Newshour Newsreel Announcement (Jim Lehrer and Charlayne Hunter-Gault) May 16 1997
http://www.pbs.org/newshour/bb/health/may97/tuskegee_5-16.html
16 Konotey-Ahulu FID. Need for ethnic experts to tackle genetic public health. Lancet 2007; 370: 1826
17 Konotey-Ahulu FID. Sickle Cell and Allied Haemoglobinopathy: The Genetics that touches you and me – University of Cape Coast, Ghana GOLDEN JUBILEE MESSAGE – http://bit.ly/1DzceM (Sept 18 2014)
18 Konotey-Ahulu FID. Tafracher – The Ghana devulgarizing prefix Personal View February 8, 1975.http://www.bmj.com/cgi/reprint/175953/329.pdf or http://www.ucc.edu.gh//node/258
The stark conclusion we can draw from the Ebola vaccine trial pug fest in Ghana is that we need to develop our own pharmaceutical industry. It is a question of national security and cultural survival. There is no other way. Reliance on western pharmaceutical companies is a sure path to extinction.












Oh mine. The more we know! $50 vs $1,700? In the same way that our leaders did not value our lives and sold some of relatives int slavery, the are selling some of our people into death! The current crop of leaders in Ghana and even since Nkrumah are pathetic bunch They read nothing, know nothing and think about nothing. Potatoes if you asked me.
All I have to say about an Ebola trial is simple: if its that good, white people should try it on white people. Simple. Then let us know how that goes. Simple.
My friend. These dogs from the West habe paid much less than 50 dollars to unsuspecting communities in Nigeria for trials that had no basis in Science or Mysticism. These pharma companies are dogs. They deserve to be slaugjtered away for bringing so much pain and suffering to innocent people.
Let me say that Alex Dodoo is a goat. A whole professor? How? This guy will poison all of Ghana by his lonesome. I am not surprised he was supportive of the illegal trials of Ebola in Ghana. The whole Mahama administration are goats and cows ruminating constantly on only fufu. No thinking, no action, no progress – goats! Beerrrrrrrr!
I agree sir! They are berrrrrrrrs! Worrying really when we still hear abt Western companies using Blacks as Guinea pigs. If its good for them, let them try it on their own kind. Simple.